Protein A ELISA Kit Performance Summary
INTRODUCTION
The Protein A ELISA Kit (catalog number 9000-1) is intended for the detection and quantitation of residual native and recombinant Protein A (rPA). Our Protein A ELISA Kit has been developed for those customers who require a highly sensitive assay to measure small amounts of contaminating Protein A in antibody products. Testing for Protein A contamination occurs in several different phases of development and commercial manufacturing which may include:
- Process development for leaching characteristics of the resin under specific conditions
- Manufacturing, typically from eluted samples taken throughout several points in the purification process
- Finish product release to document process containment levels and lot-to- lot consistency
The following summary report contains performance data collected from the evaluation of the Protein A ELISA Kit in the presence of human Immunoglobulin G (hIgG). The data presented here demonstrates the Protein A ELISA Kit’s:
- Ability to detect rPA in the presence of up to 0.5 mg/ml hIgG in a PBS-T buffer
- Percent recovery (accuracy), inter and intra assay precision, limit of quantitation and limit of detection
RESULTS SUMMARY
rPA in the Presence of hIgG
The performance of the Protein A ELISA Kit was evaluated when detecting rPA in the presence of hIgG compared to a standard containing no hIgG. All rPA spiked samples had a final hIgG concentration of 0.125 mg/ml (following final dilution into the assay plate). Each sample was prepared in replicates of 8 and three separate ELISAs were performed according to the kit’s standard protocol.
Data Handling
Standard curve data points were fitted to a 4-parameter fit analysis. This equation allowed backcalculation of sample rPA concentrations and calculation of LoQ values. Percent recovery was calculated as follows:
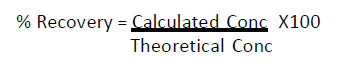
Intra-Assay Precision
Table 1. Intra-Assay Precision for Standard Curve samples (without hlgG)
| Conc (ng/ml) | Avg % CV |
|---|---|
| 1.6 | 3.7 |
| 0.8 | 3.5 |
| 0.4 | 4.7 |
| 0.2 | 6.8 |
| 0.1 | 4.6 |
| 0.05 | 8.6 |
Table 2. Intra-Assay Precision for samples containing hlgG
| Conc (ng/ml) | Calculated Conc | Avg % CV |
|---|---|---|
| 1.2 | 1.08 | 3.9 |
| 1.0 | 0.91 | 3.5 |
| 0.8 | 0.74 | 2.6 |
| 0.6 | 0.54 | 3.9 |
| 0.4 | 0.35 | 3.4 |
| 0.2 | 0.19 | 8.7 |
| 0.1 | 0.11 | 10.4 |
| 0.05 | 0.06 | 17.7 |
Inter-Assay Precision
Table 3. Inter-Assay Precision for Standard Curve samples (without hlgG)
| Conc (ng/ml) | Avg % CV |
|---|---|
| 1.6 | 3.3 |
| 0.8 | 3.2 |
| 0.4 | 4.9 |
| 0.2 | 1.6 |
| 0.1 | 4.1 |
| 0.05 | 2.1 |
Table 4. Inter-Assay Precision for sample containing hlgG
| Conc (ng/ml) | Calculated Conc | Avg % CV |
|---|---|---|
| 1.2 | 1.08 | 1.3 |
| 1.0 | 0.91 | 0.9 |
| 0.8 | 0.74 | 0.8 |
| 0.6 | 0.54 | 0.4 |
| 0.4 | 0.35 | 1.0 |
| 0.2 | 0.19 | 3.1 |
| 0.1 | 0.11 | 4.9 |
| 0.05 | 0.06 | 5.1 |
Accuracy
Table 5. Accuracy for Standard Curve samples
| Conc (ng/ml) | Avg % Recovery |
|---|---|
| 1.6 | 100 |
| 0.8 | 100 |
| 0.4 | 100 |
| 0.2 | 101 |
| 0.1 | 101 |
| 0.05 | 93 |
Table 6. Accuracy for samples containing hlgG
| Conc (ng/ml) | Calculated Conc |
Avg % Recovery |
|---|---|---|
| 1.2 | 1.08 | 90 |
| 1.0 | 0.91 | 91 |
| 0.8 | 0.74 | 92 |
| 0.6 | 0.54 | 90 |
| 0.4 | 0.35 | 87 |
| 0.2 | 0.19 | 93 |
| 0.1 | 0.11 | 106 |
| 0.05 | 0.06 | 125 |
Limit of Quanitation (LoQ)
The LoQ determined from the standard curve was calculated to be 0.037 ng/ml. The LoQ for the rPA spiked samples in the presence of hIgG was 0.102 ng/ml or 0.82 ng/mg (0.82 ppm). The sensitivity of the kit when detecting rPA50 ligand in the presence of immunoglobulin is < 1part per million (ppm) or 0.1ng/mL.
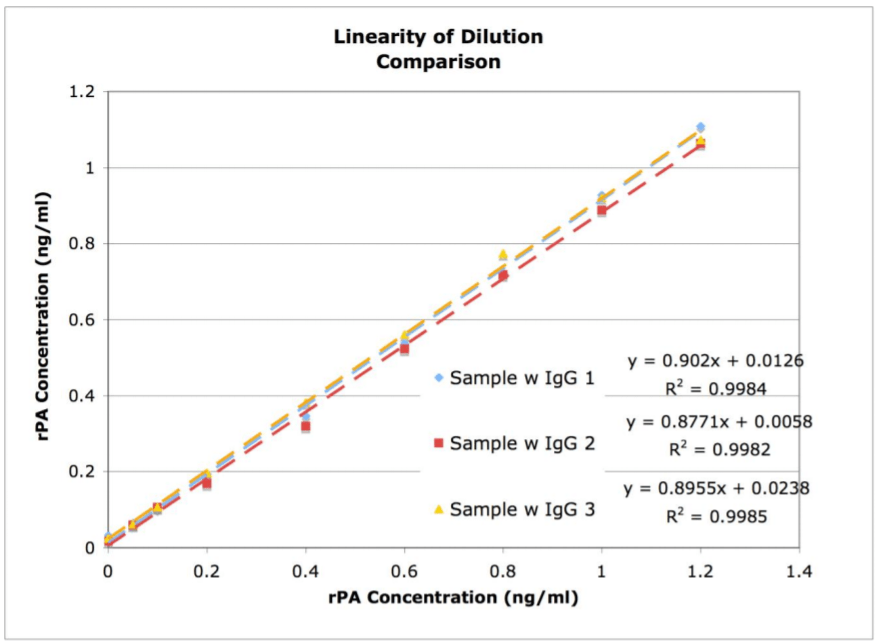
Linearity of Dilution
Figure 1.
Linearity of dilution graph showing high correlation of accuracy throughout the range of concentrations tested.
CONCLUSIONS
Intra-assay data indicated that precision for the standard curve samples were within acceptable parameters with % CV values ranging from 3.5-8.6%. The LoQ determined from the standard curve was 0.037 ng/ml.
Intra-assay data indicated that samples were assayed with relative accuracy and precision. The LoQ calculated for the samples was 0.102 ng/ml. (Sensitivity of the Protein A ELISA Kit is 0.1 ng/ml). All sample concentrations equal to and above this limit had % CV values from 10.4-2.6%, below the 15% limit.
Accuracy, gauged by average % recovery was from 93-101% for standards, and from 90- 125% for samples.
Inter-assay data indicated that data was reproducible. The coefficient of variation between assays was less than or equal to 4.9% for all standard concentrations. The samples had a high degree of inter-assay precision as well with % CV values in the range of 5.1-0.4% for concentrations equal to and above the LoQ.
EXPLANATION OF CALCULATIONS
Precision (%CV)
Precision was calculated by determining the standard deviation between rPA spiked sample data points and dividing by the mean value. According to the ‘Guidance for Industry: Bio-analytical Method Validation’ text, precision should be within 15%.
Intra-Assay Precision
The intra-assay precision was calculated for each rPA spiked sample concentration by averaging the %CV values across all assays.
Inter-Assay Precision
The inter-assay precision was calculated for each concentration point by determining the standard deviation between calculated results from each of the three assays, then dividing by the mean value.
Limit of Quantitation (LoQ)
The limit of quantitation (LoQ) was defined as 10 times the standard deviation of 0 ng/ml sample. The standard deviation of the 0 ng/ml OD value was multiplied by 10 then added to base 0 ng/ml OD value. The LoQ was then generated by entering the summed value into the standard curve 4- parameter fit equation. For each kit the LoQ was reported as ng Protein A per ml (ng/ml) buffer, and ng Protein A per mg hIgG (ppm) for rPA spiked samples run in presence of hIgG.
Limit of Detection (LoD)
The limit of detection (LoD) was defined as 3 times the standard deviation of 0 ng/ml Protein A sample. The standard deviation of the 0 ng/ml OD value was multiplied by 3 then added to base 0 ng/ml OD value. The LoD was then generated by entering the summed value into the standard curve 4-parameter fit equation. For each kit a LoD was reported as ng Protein A per ml (ng/ml) buffer and in parts per million (ppm).
Accuracy
Accuracy is described as the % recovery determined by the assay compared to the theoretical spiked concentration