Perfusion Cell Culture
Perfusion, a cornerstone of upstream continuous bioprocessing, has been practiced since the 1980s to maximize facility utilization, expand process flexibility and minimize costs. Today, novel cell retention devices and single-use technologies, with sophisticated control logic, simplify implementation.

Perfusion cell culture utilizes a cell retention device and continuous media exchange to achieve and maintain high cell densities and viabilities over extended periods of time. The cell retention device retains cells inside the bioreactor, while fresh media is added, and products of interest, waste products and spent (or depleted) media are continuously removed. Fresh media is provided at the same rate that product and spent media are removed from the bioreactor. Hollow fiber-based membrane filters act as the most reliable and commonly used membrane type. Long-term perfusion is typically performed in weeks.
Technology innovations, such as XCell ATF®, solved historical challenges, shifting the paradigm towards perfusion as a key platform for modern upstream process intensification and continuous processing.
Repligen solutions help overcome key challenges in perfusion cell culture, with hands-on process and implementation consultation from global Field Applications Specialists.

White Paper: From Fed-Batch to Perfusion: Unlocking ROI in mAb Manufacturing with Process Intensification
Fed-batch remains the traditional platform for upstream monoclonal antibody (mAb) production, but it is limited in cost efficiency, productivity, and facility utilization. As pipelines expand and demand increases, manufacturers need an upstream platform that delivers stronger process economics without compromising quality or timelines.
This white paper presents a detailed economic analysis comparing fed-batch and perfusion-based upstream production that shows perfusion with XCell® ATF Systems achieves:
- Up to 24% lower cost per gram at commercial scale
- 5–10X higher volumetric productivity (space-time-yield)
- Smaller, more flexible facilities with reduced CAPEX and OPEX
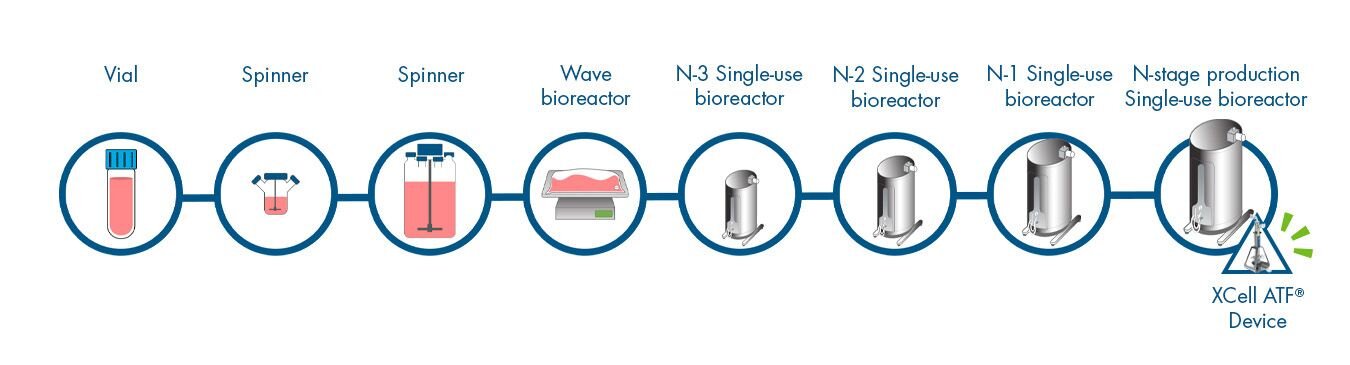
Long-term Perfusion Cell Culture Workflow
Click on the workflow link below to find productivity and throughput solutions you can implement today.
Perfusion enabled 10X VCD and 10X product than Fed-Batch
Perfusion achieved 100-130 million viable cell density (VCD) compared to 13 million for Fed-Batch. Perfusion culture viability was maintained above 90% over a period of 17 days. Fed-Batch viability began to drop after day 9 to approximately 70% at days 14-15. Higher VCD and viability of perfusion cultures translated to an increase in total product yield. The perfusion process yielded approximately 30-35 g, while the Fed-Batch process achieved 3 g in approximately 15 days.

Higher cell density, higher viability
- Unoptimized perfusion process achieved up to 10X VCD over optimized Fed-Batch
- Perfusion enabled higher cell growth rates and healthier cultures

Higher productivity
- Higher cell density and viability of perfusion culture enabled 10X yield increase over Fed-Batch

Customer First.
Support is part of the Repligen DNA. Our goal is to provide exceptional customer experience, and to support the efficient and successful adoption and implementation of all Repligen products and services.
- Field Application Support
- Customer Service
- Field Service Engineers