Solutions for mRNA / LNP Bioprocessing
With the proven success of mRNA-based vaccines in addressing the COVID-19 pandemic, mRNA technology is at the forefront of transforming the world of medicine. Clinical trials are ongoing to develop both vaccines and therapies to treat infectious diseases and even cancer.
The development and manufacturing of messenger RNA (mRNA) therapies are impacted by low process yields and scalability challenges. Vaccines based on mRNA containing lipid nanoparticles (LNPs) are a promising new platform that has demonstrated high protein expression, robust immune responses, and efficacy in numerous animal studies. Plasmid DNA (pDNA) is a critical redelivery template for mRNA.
Repligen offers solutions that help overcome key challenges in mRNA/LNP, and pDNA manufacturing, with hands-on process and implementation consultation from recognized Gene Therapy, oncolytic and vaccines industry.
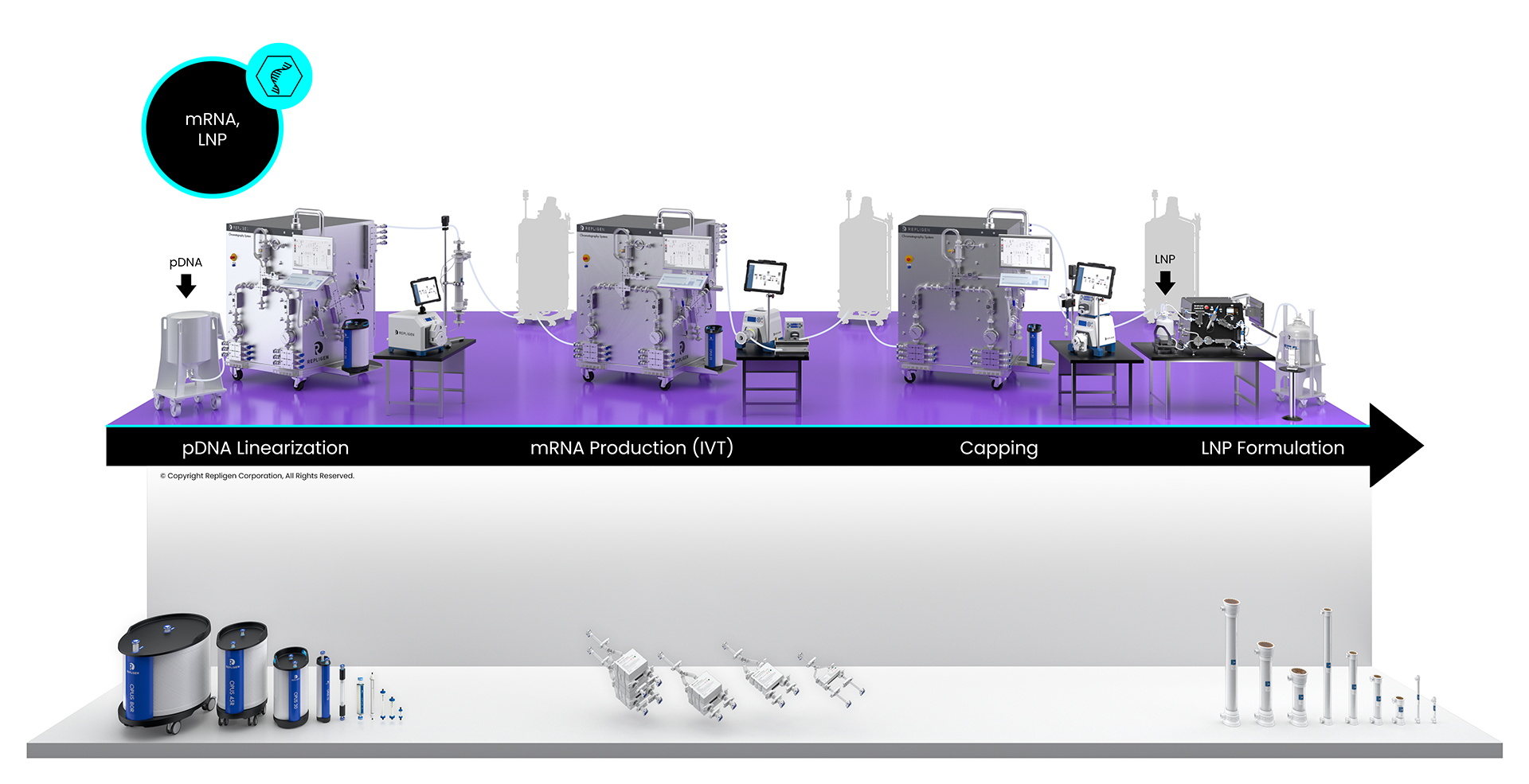
mRNA Bioprocessing Workflow
Click on the workflow links to find productivity and throughput solutions you can implement today.
Repligen workflow solutions include hardware, software and consumables that scale from process development to large-scale GMP manufacturing levels.
Challenges
Global high demand
- Scalability and cost effectiveness
- Accelerated speed to market
- Shear sensitivity
- Low productivity and purification yield
Product quality and purity
-
Removal of rDNA, dsRNA, RNA fragments, non-encapsulated LNPs
Limited process knowledge
- Limited scale-up process and implementation experience
- Lack of in-line process monitoring for cell and cell-free platforms
Repligen Solutions
Increased yield
- Optimized vector production - Simplify manufacturing process by replacing centrifugation with microfiltration TFF
- Fully scalable Chromatography and TFF equipment designed for low shear and low hold-up volume performance, maximizing product purity and recovery
Increased quality
- Fully sterile and closed automated systems with single-use flow paths
- Pre-packed columns enable purification and process consistency
- Analytical in-line technology
Expert consultation
Hands-on process and implementation approach from
recognized Gene Therapy, oncolytic and vaccines industry
experts
Effective dsRNA Removal for Safer mRNA Therapeutics
The rapid growth of nucleic acid therapeutics, such as mRNA vaccines and therapies, has introduced transformative advancements in medicine. A critical obstacle in their production is the presence of double-stranded RNA (dsRNA) impurities. These impurities can activate immune responses, compromise therapeutic safety, and reduce efficacy. Therefore, achieving efficient dsRNA removal is vital to delivering high-quality, effective RNA-based products.
AVIPure® dsRNA Clear OPUS columns are revolutionizing the dsRNA clearance process. By leveraging bead-based affinity chromatography, this innovative scavenging chromatography resin precisely separates dsRNA from single-stranded RNA (ssRNA) in flow through mode, ensuring exceptional purity and high target molecule yeilds.

- Targeted Impurity Removal
Eliminates dsRNA contaminants while maintaining ssRNA integrity, improving therapeutic safety.
- Solvent-Free Process
Avoids the use of organic solvents like ethanol, simplifying workflows and enhancing scalability.
- Industry Benchmark
Sets a new standard for dsRNA impurity clearance, making it a preferred choice for modern mRNA production processes.
With AVIPure dsRNA Clear, biopharmaceutical companies can achieve the stringent quality requirements needed for regulatory approval, paving the way for the success of RNA-based therapeutics in clinical and commercial applications.
Explore Repligen dsRNA removal solutions to enhance your mRNA manufacturing processes.
Repligen Cell and Gene Therapy
Applications Center
As a thought leader in Gene Therapy bioprocessing, Repligen maintains a state-of-the-art Gene Therapy Applications Center focused on developing internal applications expertise as well as effective collaborations with customers and Gene Therapy leaders.
The Center is staffed by a team of experts in vaccine and viral vector production, purification and analytics.


Customer First.
Support is part of the Repligen DNA. Our goal is to provide exceptional customer experience, and to support the efficient and successful adoption and implementation of all Repligen products and services.
- Field Application Support
- Customer Service
- Field Service Engineers