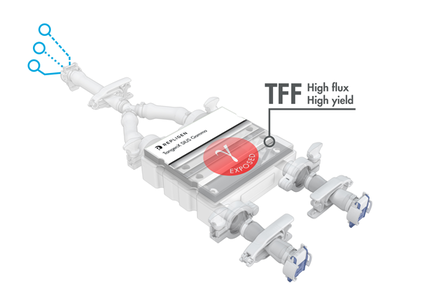
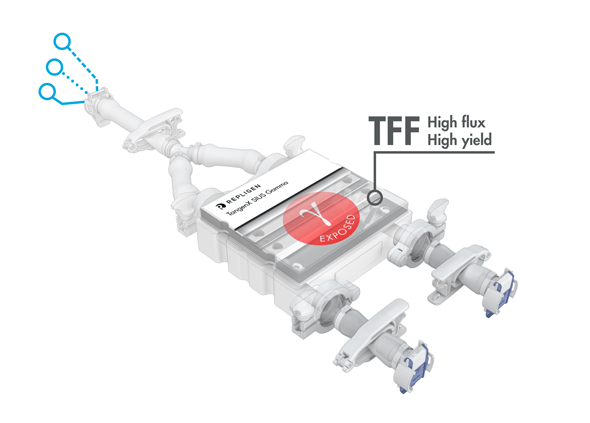
TangenX® SIUS® Gamma TFF Devices
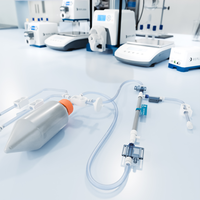
With the performance and efficiency of SIUS® Cassettes, SIUS Gamma Devices come as a convenient, fully assembled, closed, and sterile system.
Achieve higher flux, closed device and connectivity freedom
TangenX® SIUS® Gamma TFF Devices embody the performance and efficiency of SIUS® Cassettes in a convenient, fully assembled, closed, and sterile system. Acclaimed performance with up to 30% flux increase over conventional membranes, connectivity freedom, and a library of configurations, converge into one, simple TFF solution. SIUS Gamma TFF Devices are ideal for gene therapy applications, bioburden-sensitive operations, and hazardous processes.
 READY-TO-USE
READY-TO-USE
Clamp-and-go connectivity
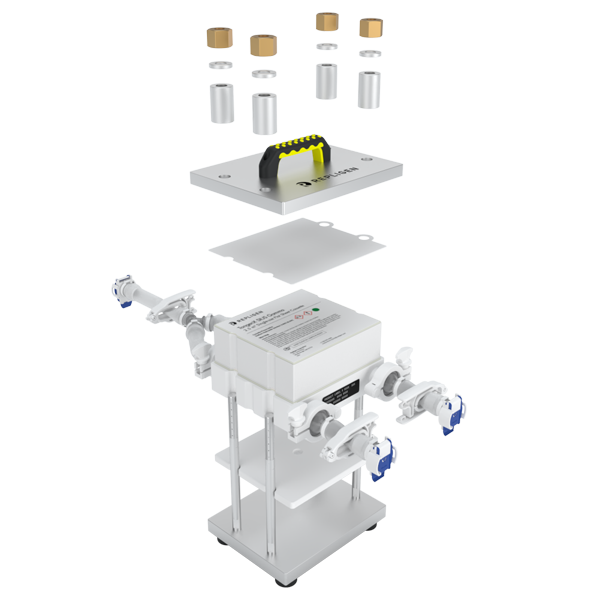
The diversity of connectivity options makes building closed systems a challenge. Genderless AseptiQuik® connectors featured on TangenX® SIUS® Gamma TFF Devices make life easier. Simply clamp the SIUS® Gamma TFF Device into a holder, connect to a flow path, and go. It's that easy.
Click on the links below to explore how to assemble SIUS Gamma TFF Devices. TangenX® SIUS® hardware may be purchased separately.
 READY-TO-USE
READY-TO-USE
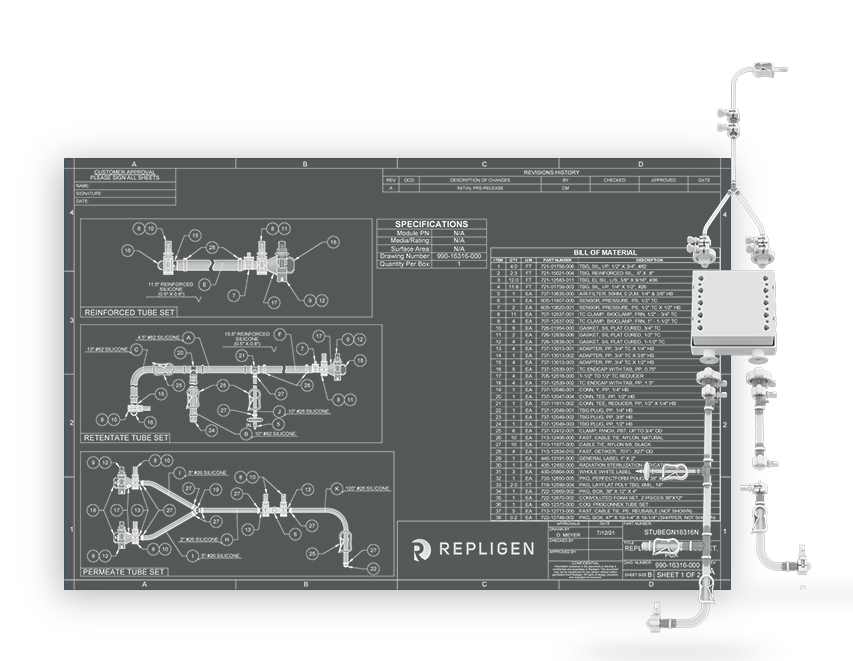
Simple connectivity with ProConnex® Flow Paths
Custom ProConnex® Flow Paths complete your TFF solution. Flow paths are build using Class VI Bioprocess-grade materials that are BSE/TSE-free and Lot-traceable.

CONFIGURED FOR YOU
Build a custom flow path to your specifications
- Engineer-designed
- Choose from over 250 components
- Connections
- Process reservoirs
- Pressure transducers
- Aseptic connectors

READY TO SHIP
Order a pre-built and quality tested off-the shelf ProConnex® Flow Path stocked for rapid delivery.
- Individually inspected
- Integrity testing available
- Clean room assembled
- Double-bagged
PLUG INTO EXISTING
Leverage the flexibility of genderless connectors and seamlessly connect to your existing flow path.
 HIGH PERFORMANCE
HIGH PERFORMANCE
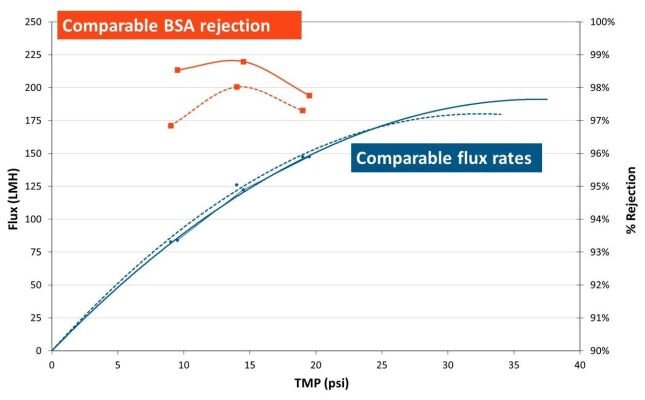
Equivalent performance with ready-to-use convenience
Transition from SIUS® Cassettes to SIUS® Gamma TFF Devices seamlessly.
Membrane performance is unaffected by exposure to gamma irradiation.
When comparing performance of TangenX® SIUS® Cassettes (solid line) and TangenX® SIUS® Gamma TFF Devices (dashed line):
- Permeate flux is comparable
- BSA rejection varies by less than 1%
 HIGH PERFORMANCE
HIGH PERFORMANCE
Diverse options for diverse process needs
TangenX® SIUS® Gamma TFF Devices are offered with two membrane chemistries, two screen types, a wide selection of molecular weight cutoffs (MWCO), and a broad range of surface areas. With 96 configurations available, TangenX® SIUS® Gamma TFF Devices accommodate diverse process requirements for ultrafiltration and diafiltration applications from process development to commercial manufacturing.

- Neutral charge
- Low protein binding
- Excellent chemical resistance
- Hydrophilic
- Low protein binding
- minimal fouling with hydrophobic species
 READY TO SCALE FAST
READY TO SCALE FAST
Scalable from lab to process scale
TangenX® SIUS® Gamma Devices scale from process development to large-scale manufacturing. Molecular weight cut-offs (MWCO)s extend from 10 kD up to 100 kD. Mermbrane area range from 0.1 - 10 m2.

Molecular Weight Cut-off
10 kD, 30 kD, 50 kD, 100 kD
Membrane area
0.1, 0.5, 1.5, 2.5, 5, 10 m2
TangenX® SIUS® Gamma Devices
TangenX® SIUS® Gamma TFF Devices incorporate the performance-leading TangenX® TFF membrane and cassette manufacturing technologies into a closed, gamma-irradiated, single-use assembly. Genderless, aseptic connections easily integrate into your current UF/DF flow path.
Click on the links below to explore different features.
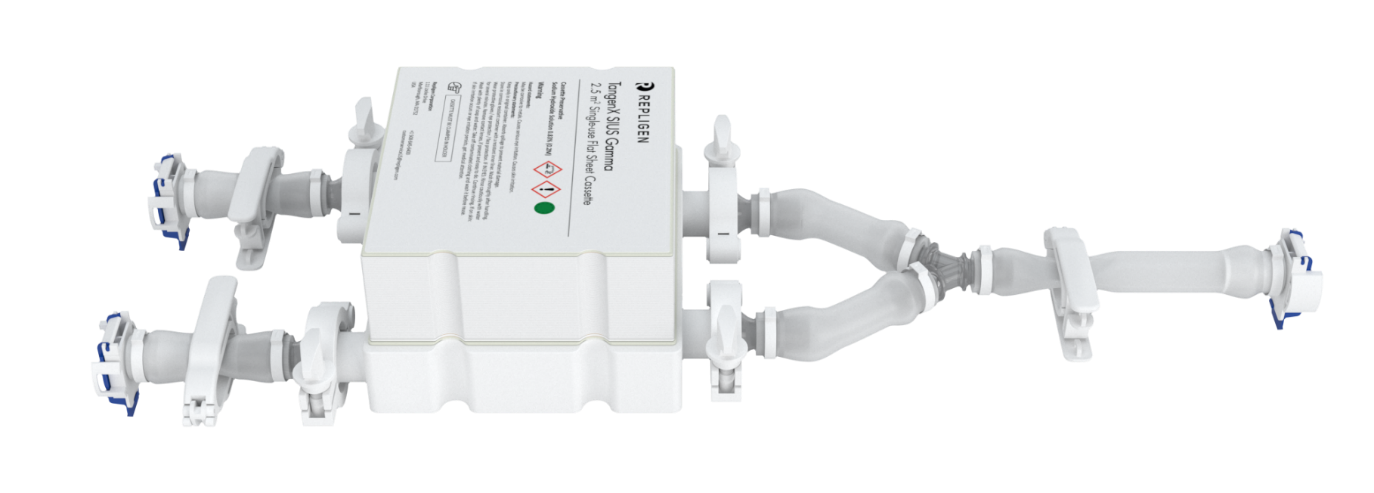


Ohhhh, snap!
In a snap, you can build a closed TangenX® SIUS® Gamma flow path. It’s that easy.
TangenX® SIUS® Gamma TFF Devices incorporate the performance-leading TangenX® TFF membrane and cassette manufacturing technologies into a closed, gamma-irradiated, single-use assembly. Genderless, aseptic connections easily integrate into your current UF/DF flow path.

Manufacturing Centers of Excellence
Repligen develops and manufactures products for the biopharmaceutical industry under an ISO 9001 quality management system. We focus on the timely delivery of high quality, consistent and robust products, to ensure business continuity for our customers.
Repligen manufacturing sites are located in Massachusetts, California, and New Jersey in the United States and in Sweden, France, The Netherlands, Germany and Estonia.

Validated manufacturing
Quality Manufacturing
TangenX® Cassettes are manufactured in a fully validated and documented manufacturing process according to the principles of cGMP. Each cassette comes with a Quality Assurance Certificate.

Comprehensive Regulatory Support Files
Each TangenX® TFF Cassette is supported by a Regulatory Support File (available upon request) that includes:
- Product information
- Cassette design
- Materials of construction
- Product performance
- Safety information
- Documentation system
- Product Manufacturing
- Qualification
- Manufacturing Process Validation
- Release Testing


Customer First.
Support is part of the Repligen DNA. Our goal is to provide exceptional customer experience, and to support the efficient and successful adoption and implementation of all Repligen products and services.
- Field Application Support
- Customer Service
- Field Service Engineers
Resources
by David Bianchi, Carl Breuning, Michael LaBreck, Shelly Parra, Mary Jo Wojtusik, Repligen Corporation
Alex Meola, Michael Mercaldi, Thomas Thiers, Homology, Medicines Inc.
Repligen Corporation
Homology, Medicines Inc.
July 2020