Innovative, differentiated technologies advancing new modalities: Case studies in mRNA & viral vectors
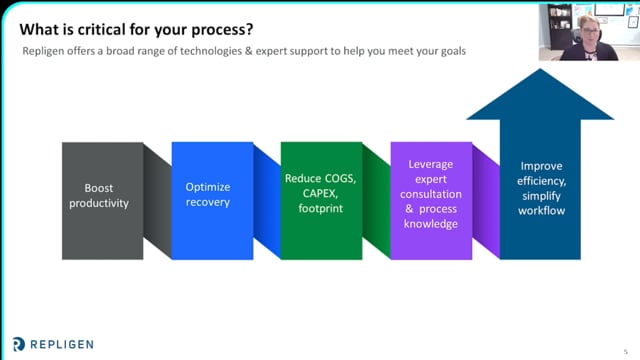
The unprecedented therapeutic potential of new modalities are accompanied by multiple new manufacturing challenges. Implementing smaller, more flexible manufacturing facilities, modalities with greater shear sensitivity, and speed to clinic, demand innovation of technologies that were largely developed for monoclonal antibodies. Innovations in upstream intensification and downstream operations that specifically benefit new modalities, like mRNA and viral vectors, are described.
Discover More Webinars
01
02
03
04
05
01
/
05