ELISA Kits

ELISA Kits from Repligen deliver the precision, reproducibility, and sensitivity required for the accurate quantitation of leached native Protein A, recombinant Protein A, the MabSelect SuRe™ ligand, the NGL-Impact® ligands, AVIPure ligands, and LONG® R3 IGF-I.
REPRODUCIBLE
ELISA Quantitation Kits
ELISA kits from Repligen are precise, reproducible and sensitive for accurate quantitation.
 ACCURATE
ACCURATE
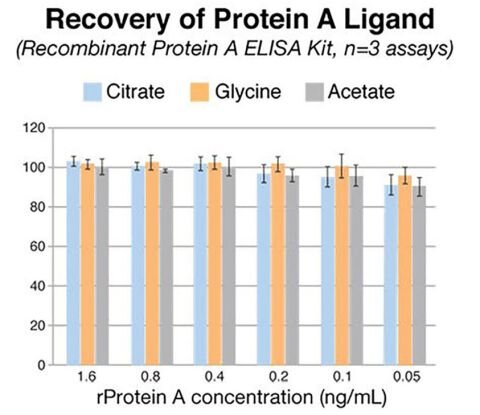
Protein A detection
Detection of leached Protein A at low limits is critical for accurate testing and reliable results. The Protein A ELISA Kits from Repligen deliver consistent recovery performance throughout the detection range of the assay across different process buffers.
 ACCURATE
ACCURATE
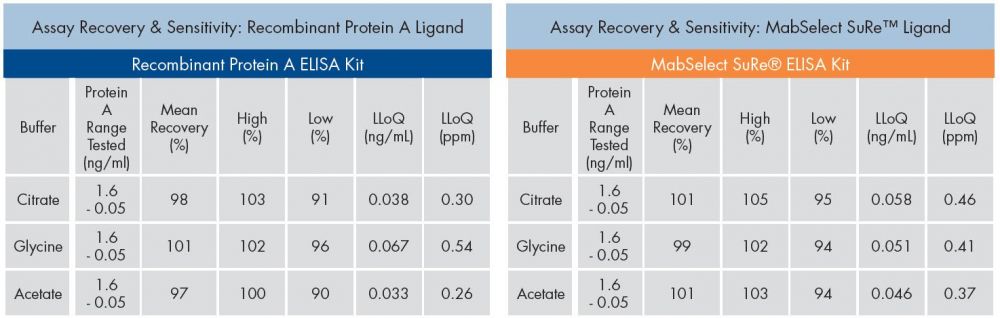
Protein A Quantitation
Although the FDA does not publish a limit on allowable levels of leached Protein A, manufacturers must characterize and quantitate the levels of Protein A contaminants in the final therapeutic formulation.
Because of their high assay recovery and sensitivity, Protein A ELISA Kits from Repligen are used in the product quality and process contaminant release testing of many monoclonal antibodies and Fc Fusion protein therapeutics.
 REPRODUCIBLE
REPRODUCIBLE
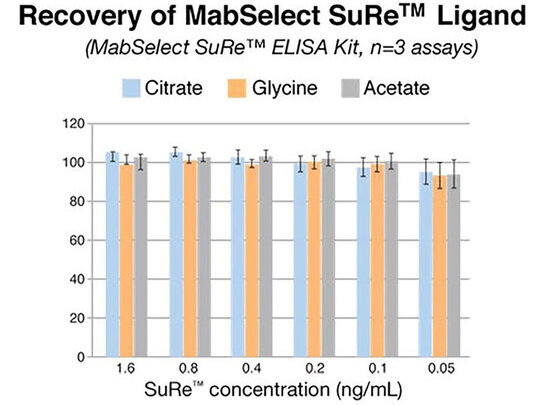
Reproducibility
The Protein A ELISA Kits from Repligen demonstrate critical inter- and intra-kit consistency. Reproducibility is demonstrated below, with the high degree of precision (% CV) throughout the dynamic range of the assay within replicate samples and across three model buffers.
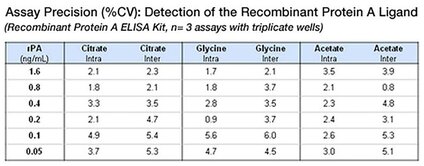
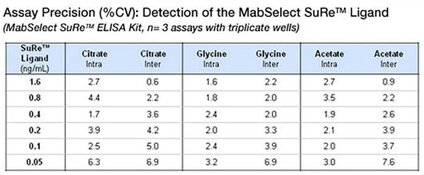
The data above were acquired using the Dilute & Go method. In all cases the sample buffer/hIgG matrix was tested in triplicate at 6 different Protein A concentration levels in three independent assays. The precision (inter and intra assay) was determined for both the Recombinant Protein A and MabSelect SuRe™ kits. The common buffers used were 100 mM Glycine, 100 mM Acetate, and 100 mM Citrate. All buffers were prepared at a pH of 3.0 and neutralized to pH 7.6 with Tris-base. Each contained 5 mg/mL of human polyclonal IgG.
 OPTIMIZED PROTOCOLS
OPTIMIZED PROTOCOLS
Sample preparation protocols
Repligen has designed three sample preparation protocols to maximize ease of use, speed, and limits of detection and quantitation.
Dilute and Go™
- Maximize ease of use and assay run time with no buffer exchange step
- Sample can be diluted directly into the assay*
Boil and Boost™
- Increase sample input concentration and decrease limit of quantitation
- Combines the low pH dissociation step with boiling (no buffer exchange step**)
Original Protocol
- Suitable for legacy methods
- Requires a buffer exchange step

Manufacturing Centers of Excellence
Repligen develops and manufactures products for the biopharmaceutical industry under an ISO 9001 quality management system. We focus on the timely delivery of high quality, consistent and robust products, to ensure business continuity for our customers.
Repligen manufacturing sites are located in Massachusetts, California, and New Jersey in the United States and in Sweden, France, The Netherlands, Germany and Estonia.


Customer First.
Support is part of the Repligen DNA. Our goal is to provide exceptional customer experience, and to support the efficient and successful adoption and implementation of all Repligen products and services.
- Field Application Support
- Customer Service
- Field Service Engineers