Gold Particle Testing
SoloVPE systems can be used for Gold particle testing, a highly effective method to validate filters and process integrity in biopharmaceutical manufacturing
Gold Particle Testing
Validation and process integrity testing for Planova filters
Virus-removal filters are a key component in many biopharmaceutical manufacturing processes.
Challenges
- The gold particle test is the most common and effective way to confirm that the filter pore size has remained unchanged throughout the process.
- Spectroscopic analysis of concentrated gold particle solutions pre- and post-filtration is the preferred method to validate chromatography filter and process integrity.
The SoloVPE Solution
- Undiluted samples are measured using the SoloVPE System to generate section data. Linear regression analysis of the section data will yield a slope value which can be used for quantitation.
- The variable pathlength technology of the SoloVPE System dynamically adjusts the measurement pathlengths to suit the sample being measured and ensures that optimal readings are made.
- The use of multiple data points provides verification that the readings are compliant with the Beer-Lambert law.
- The context of the slope measurement inherently provides a greater level of confidence than a single absorbance data point.
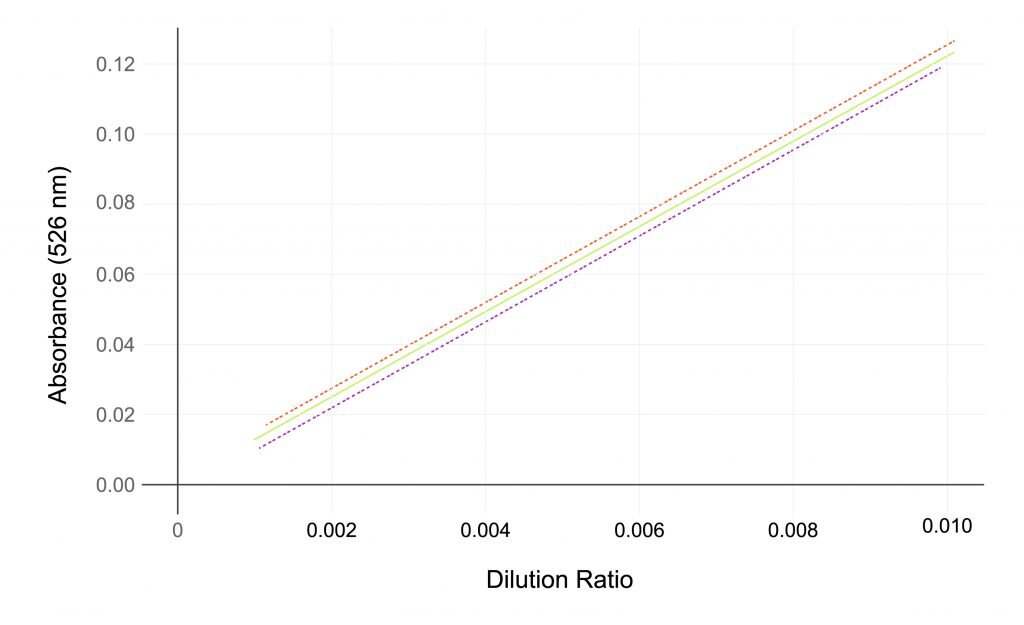
The variable pathlength technology of the SoloVPE System dynamically adjusts the measurement pathlengths to suit the sample being measured, this ensures that optimal readings are made.
Gold Particle Testing Viral Filtration Integrity Using Variable Pathlength Technology
Application Services
Your success is our success
CTech is committed to our customers’ success. We design service offerings to supplement our standard support options and provide you with increased access to our highly knowledgeable and experienced professionals. We offer implementation, development, and educational guidance to maximize the benefits of your variable pathlength solutions and the Slope Spectroscopy® technique.
Application Services
- 21 CFR Part 11 Guidance
- Annex 11 Guidance
- Validation Support
- Qualification Service
- Method Transfer Service
Development
- Method Design & Development
- Comparability
- On-Site Support
- Custom Standards
- Process Auditing
Education
- SOP Document Review
- Advanced Slope Spectroscopy Training
- Data Analysis
- Maintenance & Troubleshooting
Resources
Application Note
