Antibody Drug Conjugates
Antibody drug conjugates (ADC) represent a breakthrough in targeted cancer therapy, combining the precision of monoclonal antibodies with the potency of cytotoxic drugs. By delivering powerful treatments directly to cancer cells, ADCs provide an alternative solution that enhances efficacy and minimizes side effects.
What are Antibody Drug Conjugates?
Antibody drug conjugates (ADCs) consist of three essential components. The process begins with a monoclonal antibody (mAb) that specifically recognizes and binds to antigens on the surface of tumor cells. Unlike traditional therapies, which adversely impact all cells, the use of mAbs allows for the cytotoxic payload to only target cells that express a specific antigen that may affect all cells. This targeted approach ensures that the cytotoxic payload is delivered primarily to cells expressing the target antigen.
The payload, a highly potent drug, is designed to induce apoptosis in the targeted cancer cell upon delivery. Often 100 to 1000 times more cytotoxic than conventional chemotherapies, these payloads are uniquely suitable for targeted applications. Given their extreme potency, payloads also present significant safety risks to operators during ADC development and manufacturing.
The final component is a chemical linker that connects the antibody to the payload. The linker is vital as it must keep the payload stable during circulation and specifically release it only inside the target cell. Premature payload release can increase off target effects, potentially harming healthy cells and reducing therapeutic efficacy by lowering the effective dose delivered to the tumor. This may also contribute to the development of tumor resistance over time, complicating treatment.
Challenges and Solutions in Antibody Drug Conjugate Manufacturing
Antibody Drug Conjugate (ADC) manufacturing is a complex, multi-step process involving conjugation, filtration, purification and formulation of monoclonal antibodies, cytotoxic payloads, and chemical linkers.
Key challenges include
- Managing potency of the payloads
- Minimizing cytotoxin exposure to operators
- Ensuring consistent drug-to-antibody ratios (DAR) across batches
- Containment and storage
From early-stage development to commercial production, Repligen offers integrated solutions across key unit operations, including antibody production, conjugation, drug-linker preparation, closed system connectivity, and ADC formulation. These solutions enable optimization of ADC production processes for improved yields, product quality, operator safety and cost-effectiveness.
Antibody–Drug Conjugate Manufacturing: Driving Process Productivity and Reducing Risk
ADC manufacturing remains a complex, multi-step process with inherent challenges. Both early- and late-stage manufacturers must navigate the intricacies of handling highly potent payloads, maintaining consistent drug-antibody ratios (DAR), minimizing operator exposure, and ensuring product efficacy and safety. Hear from expert Field Applications Scientists as they discuss technologies that can help to:
- Accelerate processing time
- Maintain product consistency and operator safety
- Drive efficiency with accurate, real-time DAR measurement

 Start the Journey: Achieving Safety, Efficiency, and Flexibility in ADC Manufacturing
Start the Journey: Achieving Safety, Efficiency, and Flexibility in ADC Manufacturing
From theory to therapy- Immerse yourself in the world of antibody-drug conjugates (ADCs) in our latest white paper. Learn about the anatomy of ADCs, unique manufacturing challenges, and the critical role of single-use systems in ensuring safe, efficient, and flexible production processes. Glean insights and discover the operational advantages Repligen Tangential Flow Filtration systems and Chromatography systems offer to ADC manufacturers.
.jpg) Dig into the Data: Extractables Study on Single-use Consumables for ADC Production
Dig into the Data: Extractables Study on Single-use Consumables for ADC Production
When adopting single-use systems for ADC manufacturing, added emphasis is placed on the risk of extractables and leachables (E&L) from the flow kits and membranes. These substances, which may be released under certain conditions, could potentially impact the safety and efficacy of the final product. Our study delves into this concern, evaluating E&L from ProConnex® Flow Paths and TangenX® SIUS Gamma Cassettes, particularly in the presence of common solvents like dimethyl sulfoxide (DMSO) and dimethylacetamide (DMAC).
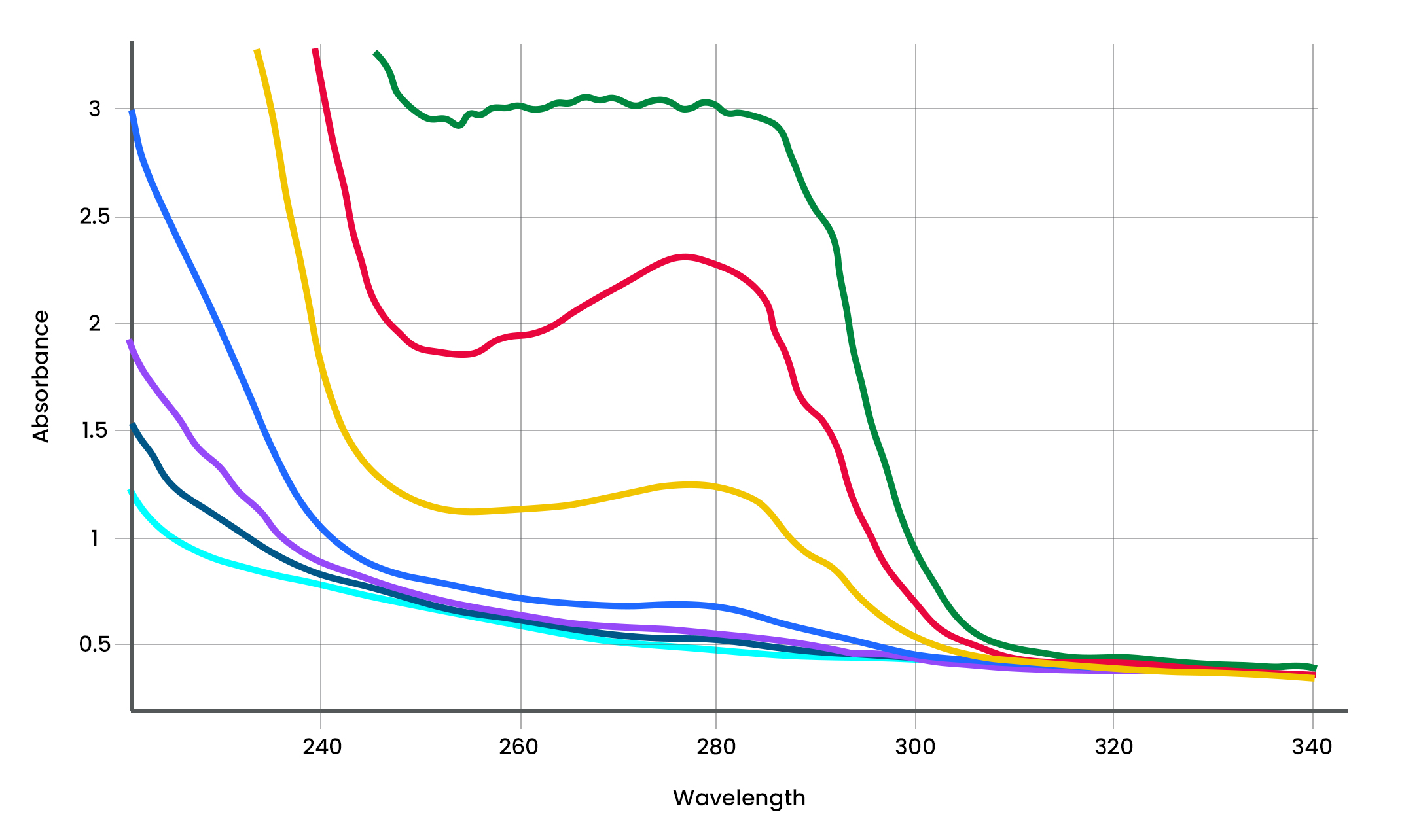 Analyze the Findings: Validation of ADC Platform for Drug-Antibody Ratio (DAR) using Variable Pathlength Technology
Analyze the Findings: Validation of ADC Platform for Drug-Antibody Ratio (DAR) using Variable Pathlength Technology
Critical to the design and evaluation of ADCs during formulation, Drug-Antibody Ratio (DAR) refers to the number of drug molecules attached to the antibody. But with several DAR measurement methods to choose from, which provides the greatest degree of accuracy and actionable insights? Discover the superiority of the variable pathlength technology that powers our CTech™ SoloVPE® PLUS and FlowVPX® systems in this insightful analytical review.
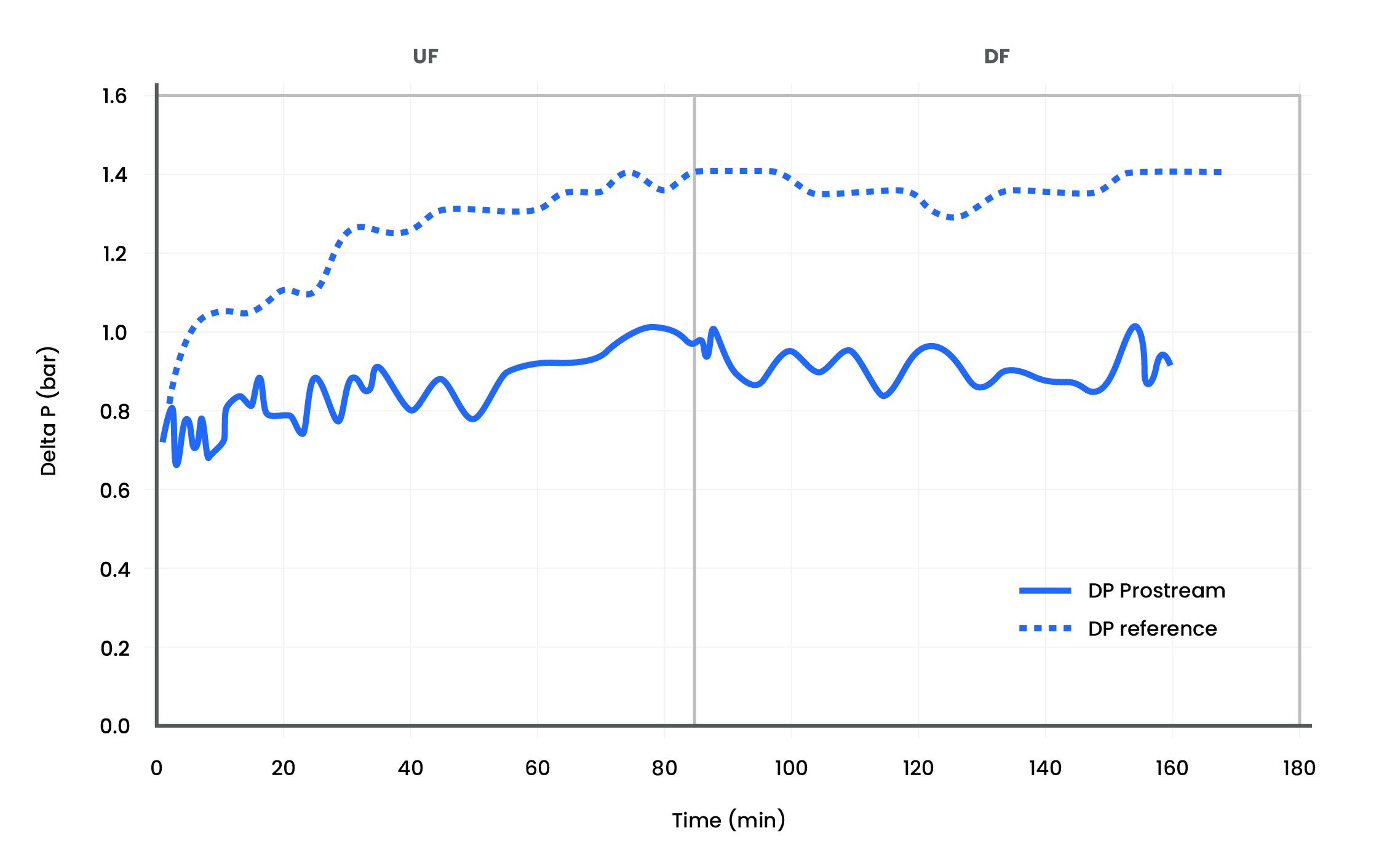 Collaborate with Confidence: Review of Single-Use Flat Sheet Cassettes in ADC Processing with Sanofi
Collaborate with Confidence: Review of Single-Use Flat Sheet Cassettes in ADC Processing with Sanofi
When it comes to ADC purification, performance matters. Discover how Repligen’s TangenX® ProStream™ and HyStream™ tangential flow filtration (TFF) cassettes deliver high-efficiency output across critical ultrafiltration and diafiltration steps in ADC production. Developed in collaboration with Sanofi, this in-depth study showcases real-world data on solvent clearance, membrane integrity, and product recovery.