HiPer™ QA Resin

Designed for the purification of extra-large biotherapeutic molecules, the HiPer™ QA Resin achieves superior resolution of full vs. empty AAV capsids. Using innovative macroporous bead technology, this resin delivers high dynamic binding capacity, ultra-fast processing, lot-to-lot consistency, and high scalability to accelerate speed to market.
Superior resolution
- Outperforms leading commercial polish resin
- Achieves full/empty capsid separation
- Supports safety and efficacy in gene therapy manufacturing
High DBC for rapid processing
- 1-2 µm pores for target molecules larger than 20 nm
- Loading amount ≥ 1014 VP/mL
- Only 15 seconds of residence time
Designed for scalability
- Consistent performance independent of flow rate
- Lot-to-lot consistency
- Scalable from process development to manufacturing
High Dynamic Binding Capacity
HiPer™ resins use an innovative macroporous bead structure, which allows for convective flow both through and around the beads, resulting in high binding capacity at fast flow rates. Unlike traditional resins, the HiPer™ QA resin features a superior loading amount (e.g., ≥1E14 AAV capsids/mL) at high flow rates, outperforming the leading competitor.
With 1─2 µm pore sizes, this resin suitable for target molecules larger than 20 nm. The large pores are ideal for the purification of large-molecule biologics, such as viral vectors, viruses, and nucleic acids.
Modalities:
- Viral vectors: Adeno-associated virus (AAV), Lentivirus, Adenovirus, virus-like particles (VLP)
- Viruses: Influenza
- Nucleic acid therapeutics: mRNA, plasmid DNA
- Extracellular vesicles
- Exosomes

Generally, full AAV capsids, which contain the target AAV genome, are more negatively charged than empty capsids. This difference allows for selective binding and elution of the capsids using anion exchange chromatography. However, empty capsids can still elicit an immune response, creating challenges for safety and efficacy in gene therapy.
Unlike other available resins on the market, the HiPer™ QA resin is designed specifically for the polish step of AAV manufacturing and achieves notable separation of full vs. empty (or partially filled) capsids. Effective capsid separation enables manufacturers to maintain high target molecule yield while ensuring product safety and efficacy.
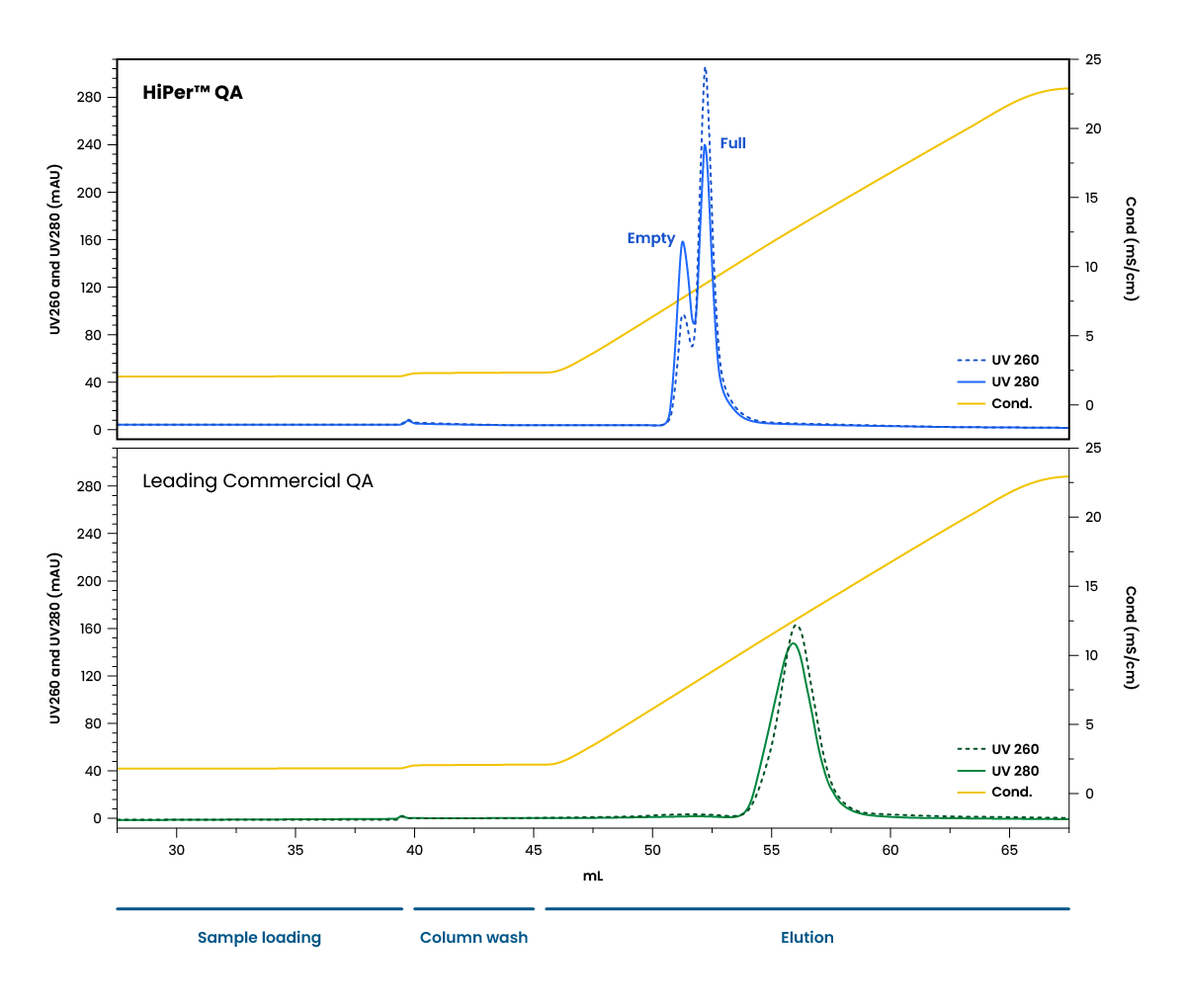
The HiPer™ QA resin was tested against a leading commercial QA resin for polish. The AAV8 capsids were first bound during the loading step. Empty and full capsids were sequentially eluted as conductivity increased during the elution step. In contrast, the commercial resin did not successfully separate empty and full capsids, instead resulting in co-elution.
AAV capsids are prone to degradation when exposed to low-conductivity buffers or prolonged retention inside the column. Minimizing column residence time—and therefore the exposure to low-conductivity buffer conditions—can mitigate the risk of capsid degradation. HiPer™ QA resins achieve capsid separation with as short as 15 seconds of residence time. Resin performance remains consistent across flow rates, allowing manufacturers to process quickly and preserve precious viral capsids. This high processing speed also supports process productivity to help bring therapeutics to market faster.
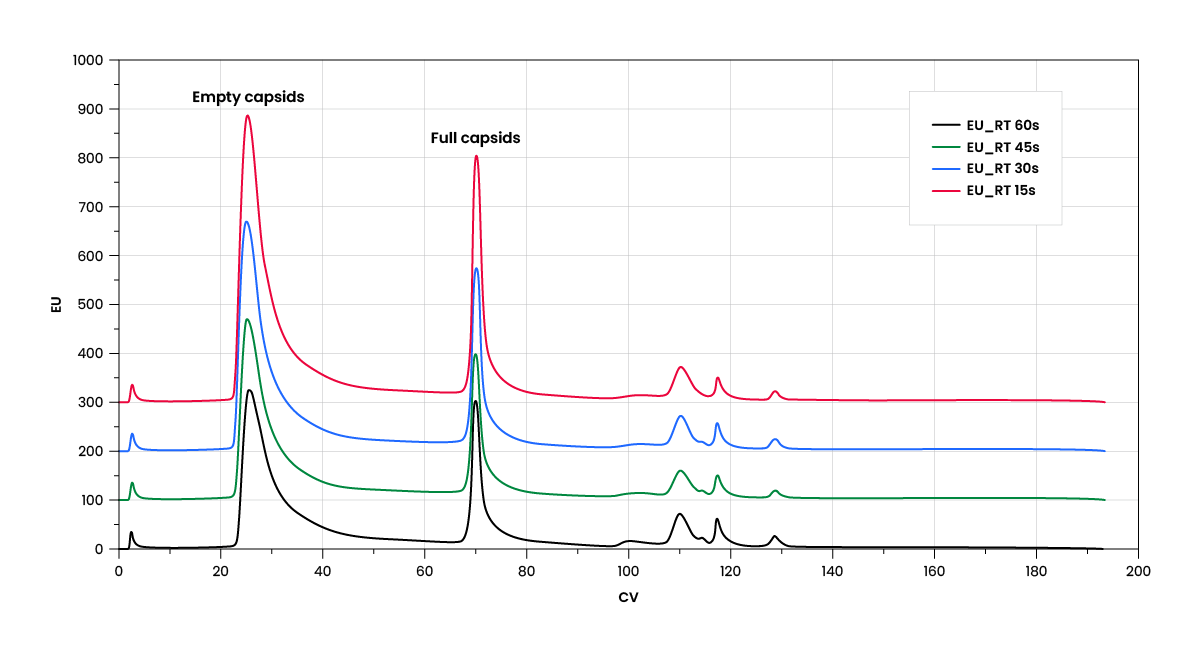
HiPer™ QA resin performance across flow rates at 15-, 30-, 45-, and 60-sec residence times. Performance is unaffected by flow rate, maintaining excellent separation efficiency with no obvious degradation of AAV capsids.
Variation in performance attributes across different lots of QA resin requires costly redevelopment of purification methods. Therefore, consistency between resin lots is crucial. As demonstrated here, performance and conductivity of the HiPer™ QA resin is consistent across lots. Purification conditions developed using one lot can be applied to future batches, translating to reduced development costs for future processes.
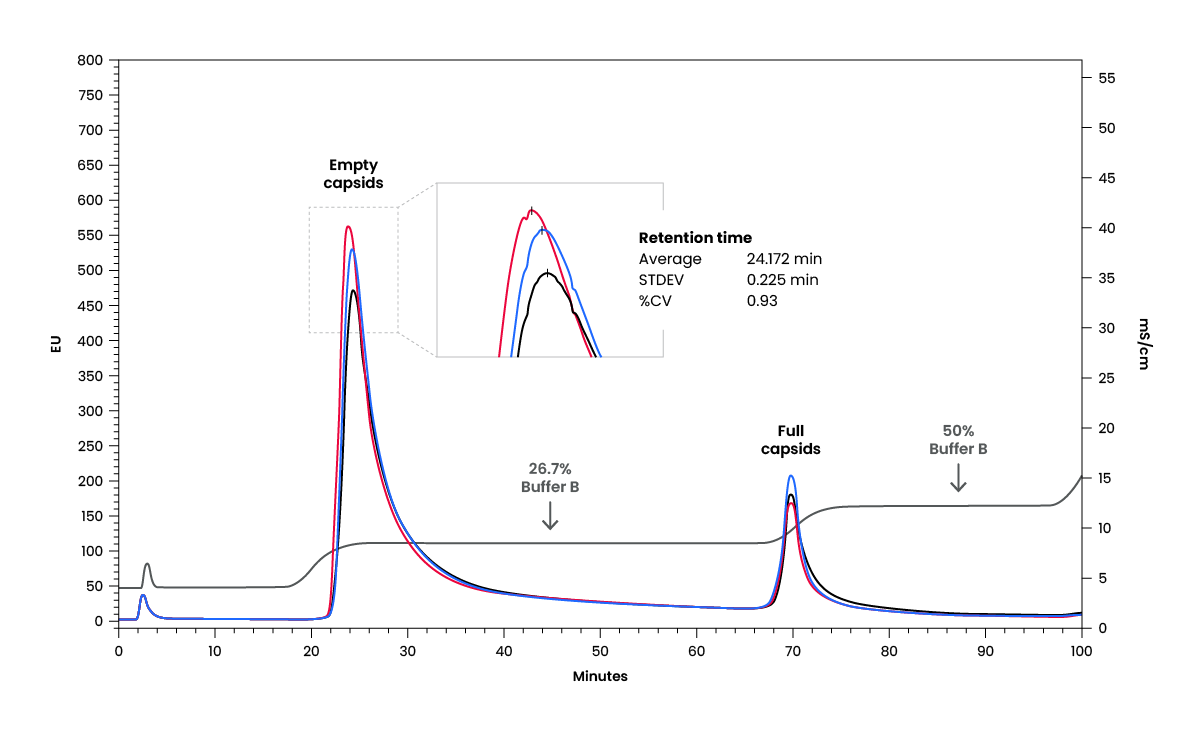
To verify batch consistency, three different lots of the HiPer™ QA resin were tested. First, HiPer™ QA resin was selected from one of three lots and separation of empty and full AAV capsids was performed using a linear elution method. Then, a two-step elution was set up basing on the elution conductivity of empty full AAV capsids from the chromatogram of linear elution. When the same step elution method was applied to columns packed with two other PV lots, we observed similar empty & full capsids retention times, full capsids purity (A260/280), and yield (full capsid peak integration).
Designed for Scalability and Stability
HiPer™ QA resins are designed to scale easily from process development to commercial manufacturing. Efficient mass transfer, high target molecule yield, and fast processing support improved process efficiency through accelerated validation.
The resin’s high salt tolerance not only enables target binding under higher conductivity conditions, but also helps prevent increases to sample volume and purification time caused by sample dilution. The HiPer™ QA resin can also withstand up to 15 hours in 1M NaOH at ambient temperature, or 30 minutes of contact time per cycle for 30 uses, making it ideal for GMP-compliant manufacturing environments.
Maximize Yield and Efficiency
Traditional polish methods often require a balance between maximizing target molecule yield via affinity chromatography and achieving effective separation in a subsequent polishing step. By pairing the HiPer™ QA resin with next-generation AVIPure® AAV affinity resins, manufacturers can now achieve both high target molecule yield and high separation efficiency simultaneously.
Available in OPUS® Pre-Packed Chromatography Columns
HiPer™ QA resins are exclusively available in OPUS® pre-packed chromatography columns for convenience, consistency, and speed. Columns are customizable to meet your specifications, with short lead times to keep your project on track.

Accelerate Your Process Development
Explore the HiPer™ QA resin today and enhance your process productivity with rapid, effective purification.
Related Products