Fluid Management

Repligen offers innovative, designed-for-purpose storage and transfer solutions for fluid management within and between unit operations. These include flow paths, tubing, valves and gaskets, bottles and containers, totes, carts and cleanroom process equipment.
Fluid management is critical within and between every unit operation in upstream and downstream biopharmaceutical manufacturing processes. Each touchpoint, regardless of size, presents a risk of failure as well as an opportunity for step advances in process economics and process efficiency.
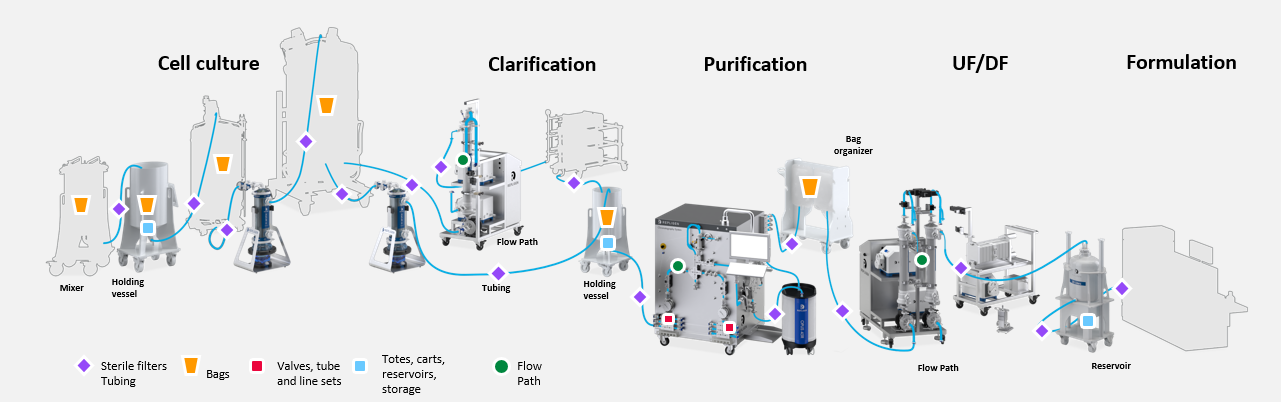
Repligen fluid management solutions include state-of-the-art single-use components, and innovative storage and transfer equipment.
Single-use components
- Conveys fluid across unit operations
- Includes equipment and consumables
- Grows, processes and conveys biotherapeutics and process liquids
Storage and transfer
- Connects the unit operations and stores material
- Includes bags, bottles, tubing, totes and other components
- Excludes processing equipment


Non-metallic Process Equipment
Repligen non-metallic process equipment are cost-effective alternatives to traditional stainless steel totes and carts. With virtually unlimited options and configurations, Repligen products are made of highest quality Class VI TSE/BSE free materials and customized to the customer's specific application.


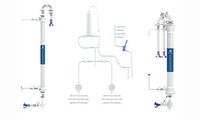
ProConnex® Flow Paths
Expertly designed plug-and-play flow path
ProConnex® Flow Paths for TFF and Chromatography processes are single-use and engineered for optimum performance, reproducibility and time savings. System specific flow paths provide speed and reliability. Configured flow paths prioritize process requirements and sterility.

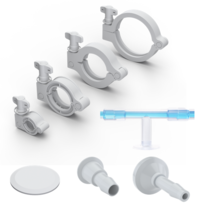
Connectivity
Security at the point of connection
Hundreds of connection points are present throughout your workflow, each presenting risk of failure. ProConnex® single-use connectivity solutions are purpose-built for bioprocessing applications, optimizing fluid transfer within and between critical process steps.
