XCell® ATF Systems
SIMPLIFY AND INTENSIFY
XCell® ATF Systems attach to a bioreactor to intensify upstream manufacturing capacity, productivity, and throughput.
 Intensification Leader
Intensification Leader
Intensification delivers more product faster
XCell ATF Technology helps simplify and intensify upstream processes to deliver more product, faster. Intensified processes achieve higher cell densities, require smaller bioreactors and consume less suite time. Increase throughput, productivity, and capacity in both clinical and commercial manufacturing.

White Paper: From Fed-Batch to Perfusion: Unlocking ROI in mAb Manufacturing with Process Intensification
Fed-batch remains the traditional platform for upstream monoclonal antibody (mAb) production, but it is limited in cost efficiency, productivity, and facility utilization. As pipelines expand and demand increases, manufacturers need an upstream platform that delivers stronger process economics without compromising quality or timelines.
This white paper presents a detailed economic analysis comparing fed-batch and perfusion-based upstream production that shows perfusion with XCell® ATF Systems achieves:
- Up to 24% lower cost per gram at commercial scale
- 5–10X higher volumetric productivity (space-time-yield)
- Smaller, more flexible facilities with reduced CAPEX and OPEX
 INTENSIFICATION LEADER
INTENSIFICATION LEADER
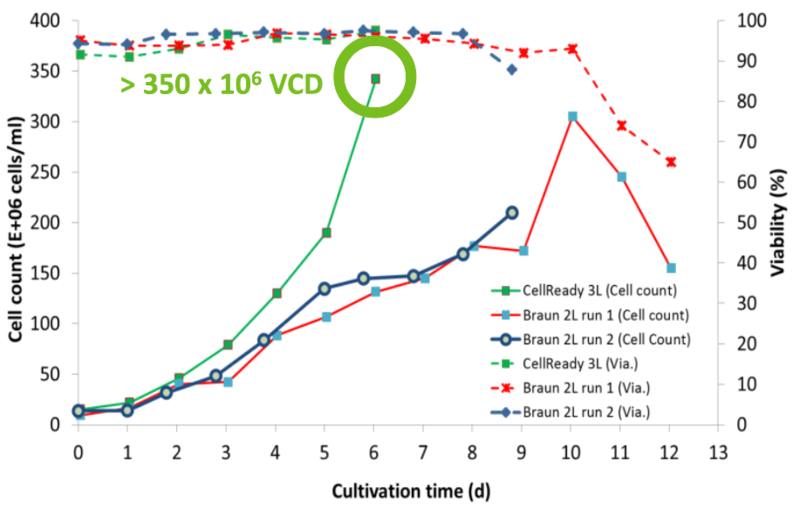
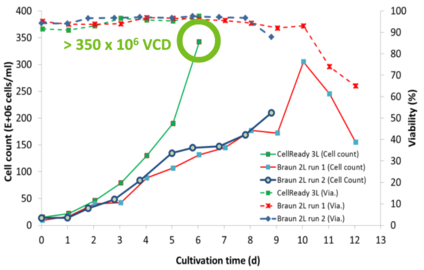
XCellATF delivers 10X VCD and lower COGS
Higher productivity and lower COGs for malaria therapeutic candidate.
- Can deliver 10X VCD more than 2-week Fed- Batch (350 x 106 cells/mL)
- 50% decrease in bioreactor time
- 20-fold increase in yield
 Breakthrough technology
Breakthrough technology
How XCell ATF Technology Works
The XCell ATF System is based on award-winning alternating tangential flow (ATF) technology, created by the action of a diaphragm moving upward and downward within a pump head, connected to a filter housing and attached to a bioreactor. Alternating tangential flow is attained by the action of a diaphragm pump.

 Breakthrough technology
Breakthrough technology
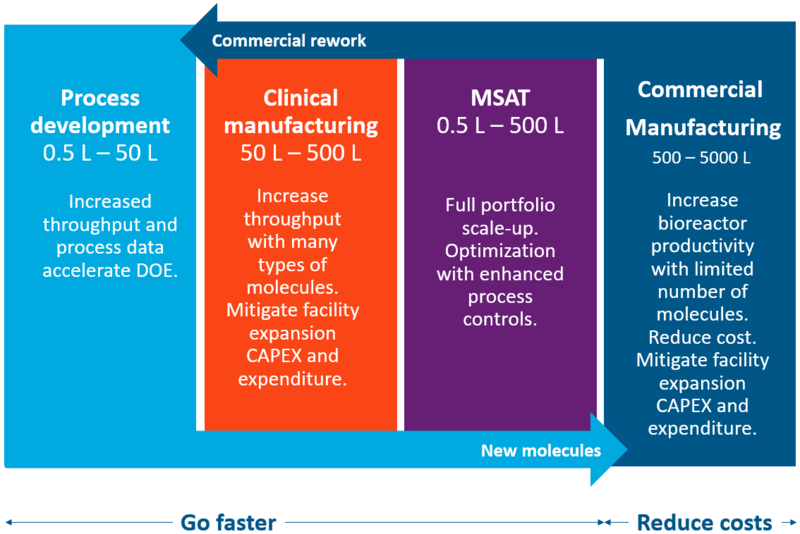
Ready to scale from process development to manufacturing
The XCell ATF System simplifies and fast-tracks upstream intensification development from PD to commercial scale. Intensification increases throughput, accelerates tech transfer and optimizes your process in clinical manufacturing and MSAT while increasing bioreactor productivity and reducing costs in commercial manufacturing. Higher productivity, smaller bioreactors and higher throughput with shorter times to harvest, combine to mitigate facility expansion risks and capital costs.
 Integrated system
Integrated system
Design simplicity with XCell® Lab System
Designed and engineered as a complete solution, the XCell Lab System integrates a modern controller, XCell ATF Devices, software and flow sensors into a complete solution.
- Engineering-designed controller promotes increased intensification
- Configurable set-up delivers high throughput - a single manifold supports up to 8 XCell ATF Devices
- Software enables precise technical control with a user-friendly interface
- New XCell ATF 1 Device is fit for low volume intensification ready for scale-up
- Repligen global technical support ensures successful implementation and scale-up

XCell® LS Controller for Pilot and Commercial Manufacturing
Scaling up from bench scale to commercial scale can be simple with a reliable controller for vital cGMP operations
- Pairs with single-use and stainless steel XCell ATF 6 and XCell ATF 10 devices, and the stainless steel XCell ATF 4 device
- Single LS Controller controls single or dual ATF devices at once
- Advanced process monitoring: flow sensor-based control, permeate pressure sensing/filter fouling
- Intuitive software with touchscreen interface, 21 CFR part 11 compliant, and Windows 10 Domain security
- Operate independently with HMI or integrate with site DCS systems (headless configuration)
- Global technical support ensures successful implementation and scale-up

XCell ATF Devices and Controllers
The XCell ATF System delivers a complete solution for upstream intensification with robust hardware software integration. Available in single-use and stainless steel formats with sizes from lab-scale (2 L) to process scale (5000 L). Each system consists of an XCell ATF Device, XCell Controller, software, tubing and sensors.

XCell ATF Systems
XCell ATF Cell retention technology simplifies and fast-tracks upstream intensification development for gene therapy, fed-batch intensification, seed train intensification, perfusion, and media exchange applications.



The XCell™ Lab Controller solves your productivity puzzle with new engineering, increased throughput and more process data. Turn the frustration of endless permutations into a simple solution that generates more cells, and more product…faster.

Intensification is worth it. Build an intensified upstream suite brick by brick with XCell ATF® systems. Make twice as much product in half the time as you watch VCD grow and shrink bioreactor sizes within the same facility footprint.

OMG life gets busy. Set-up industry-best cell culture intensification in less than 30 minutes with the design simplicity of XCell ATF® devices. Celebrate more product at work…and still have time for the special moments at home. YGTI.
Manufacturing Centers of Excellence
Repligen develops and manufactures products for the biopharmaceutical industry under an ISO 9001 quality management system. We focus on the timely delivery of high quality, consistent and robust products, to ensure business continuity for our customers.
Repligen manufacturing sites are located in Massachusetts, California, and New Jersey in the United States and in Sweden, France, The Netherlands, Germany, France and Estonia.


Customer First.
Support is part of the Repligen DNA. Our goal is to provide exceptional customer experience, and to support the efficient and successful adoption and implementation of all Repligen products and services.
- Field Application Support
- Customer Service
- Field Service Engineers
Resources
Investigation of XCell ATF® Perfusion Technology for virus manufacturing process intensification at MSD Animal Health
Repligen Corporation
MSD Animal Health
July 2022
Specifications
 |
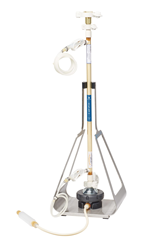 |
 |
 |
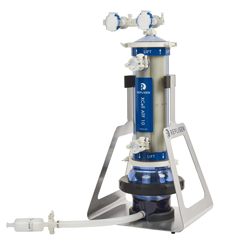 |
||
|---|---|---|---|---|---|---|
|
XCell® ATF 1 |
XCell® ATF 2 |
XCell® ATF 4 |
XCell® ATF 6 | XCell® ATF 10 | ||
| Typical Bioreactor size (L) | 0.5 - 2 | 2 - 10 | 10 - 50 | 50 - 200 | 200 - 5000 | |
| Format | SU | SU, SS | SS | SU, SS | SU, SS | |
| Chemistry | PES | PES, PS | PES, PS | PES, PS | PES, PS | |
| Typical pore size SU | 0.2µ | 0.2µ | 0.2µ | 0.2µ | 0.2µ | |
| Typical pore size SS | NA | 0.2µ, 0.5µ, 50KDa | 0.2µ, 0.5µ, 50KDa | 0.2µ, 0.5µ, 50KDa | 0.2µ, 0.5µ, 50KDa | |
| Effective surface area (m2) | 0.022 | 0.13 | 0.77 | 2.5 | 11 | |
| Filter height (cm) | 60 | 60 | 30 | 60 | 60 | |
| Displacement volume (L) | 0.017 | 0.1 | 0.4 | 1.3 | 6 | |
| ATF flow range (Lpm) | 0.008 - 0.140 | 0.4 - 1.5 | 3 - 8 | 10 - 20 | 30 - 80 | |
| Typical scalable flow/fiber and filtrate rate | 12 mL/min/fiber and 4-6 LMH | |||||
| Controller | Lab Scale Controller |
Lab Scale Controller |
||||